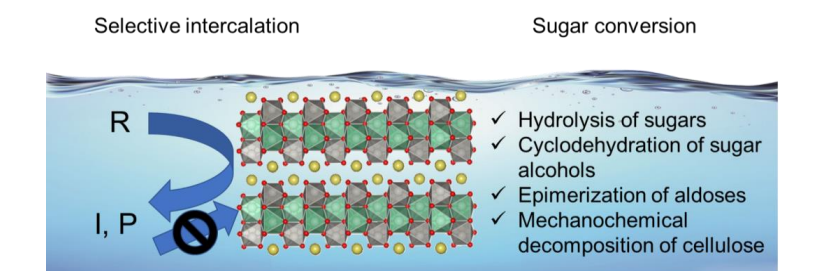
1.The acid-base regulation function of surface hydroxyl groups
Hydroxyl groups (- OH) can exhibit acidity or alkalinity on the surface of metal oxides in the form of proton reception or supply. By adjusting the quantity and distribution of hydroxyl groups, precise control of surface acidity and alkalinity can be achieved, thereby affecting the activation pathway and selectivity of catalytic reactions.
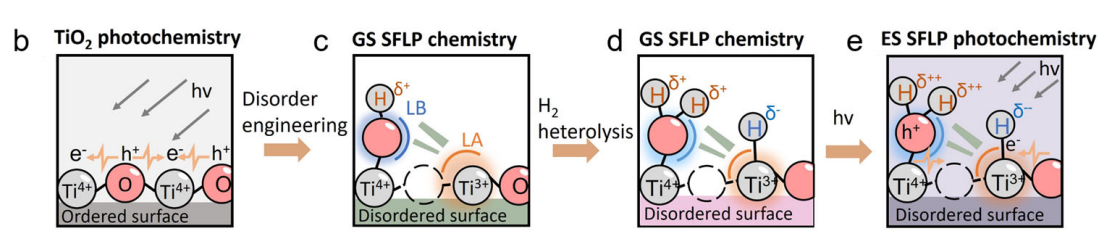
2.The influence on electronic structure and adsorption behavior
The presence of hydroxyl groups can alter the local electron density on the surface, thereby regulating the electronic structure of active sites. Density functional theory (DFT) simulations indicate that different hydroxyl densities (such as bridging hydroxyl and pseudo bridging hydroxyl) lead to significant differences in surface bonding energy and charge distribution, which directly affect the adsorption strength and activation energy of substrate molecules.
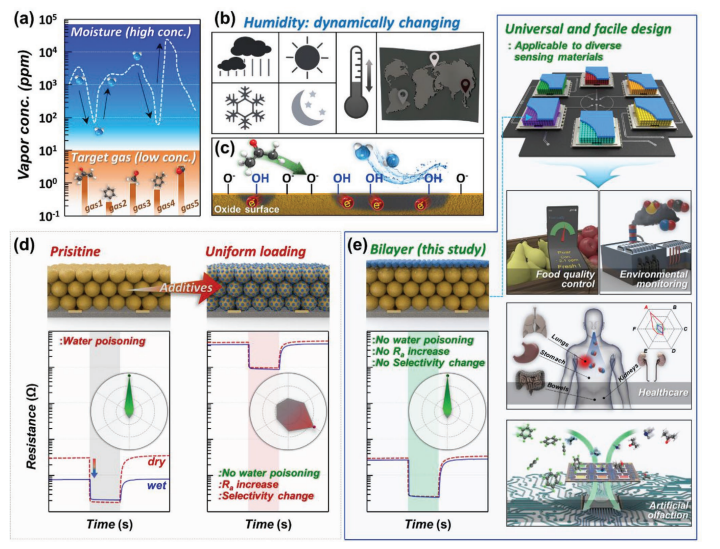
3.Toxic mechanism caused by adsorption of water molecules
In the actual reaction environment, water molecules will adsorb and dissociate to form surface hydroxyl groups, forming "water poisoning". These newly generated hydroxyl groups will occupy the original active sites (such as oxygen vacancies), hindering the regeneration of oxygen vacancies and leading to a rapid decline in catalyst activity.
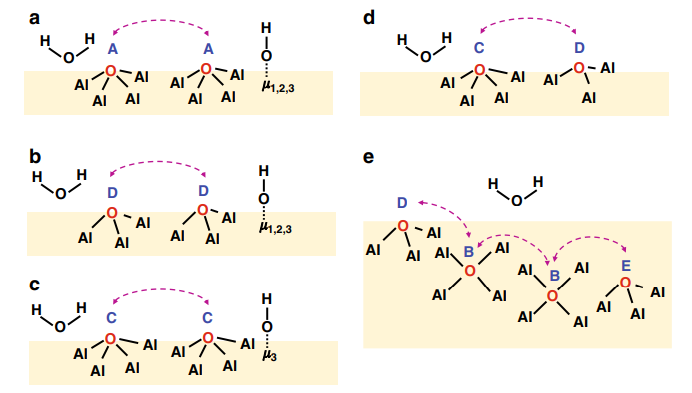
4.Fine control of hydroxyl density and spatial distribution
The spatial arrangement of hydroxyl groups (bridging, pseudo bridging, individual hydroxyl groups) determines the geometric and chemical properties of the surface structure. By adjusting the coverage of hydroxyl groups, systematic control of surface polarity, hydrophilic/hydrophobic equilibrium, and thermodynamic stability of catalysts can be achieved.
5.Protection and reactivation of active sites
By surface functionalization or introducing hydrophobic molecules, the excessive accumulation of hydroxyl groups can be selectively eliminated or prevented, thereby protecting the active sites and restoring the cyclic use of oxygen vacancies. This type of engineering method can significantly enhance the intrinsic activity of transition metal oxide catalysts.
SAT NANO is a best supplier of nano particle and micro particle in China, we can offer carbon nanotube powder, such as
MWCNT-COOH,
MWCNT-OH, if you have any enquiry, please feel free to contact us at sales03@satnano.com





























