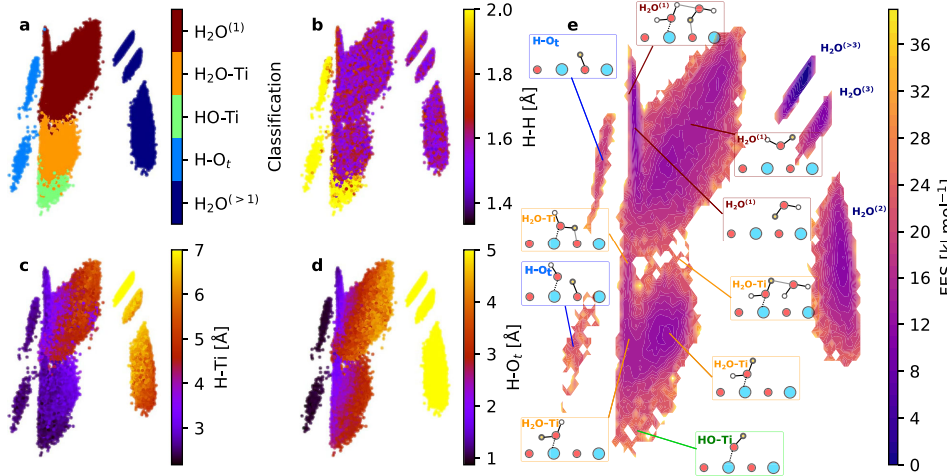
1.Direct dissociation and adsorption of water molecules
On unsaturated metal sites of metal oxides or semiconductor oxides (such as Ti4+, Fe3+), water molecules first adsorb in molecular form, followed by O-H bond cleavage, resulting in bridge or terminal hydroxyl groups (M-OH) and surface hydrogen atoms. The thermodynamic driving force of this process comes from the strong Lewis acidity of metal ions, making water molecules easy to dissociate. Both experiments and DFT calculations indicate that surfaces covered with low oxygen tend to dissociate and adsorb, while surfaces covered with high oxygen tend to adsorb molecules.
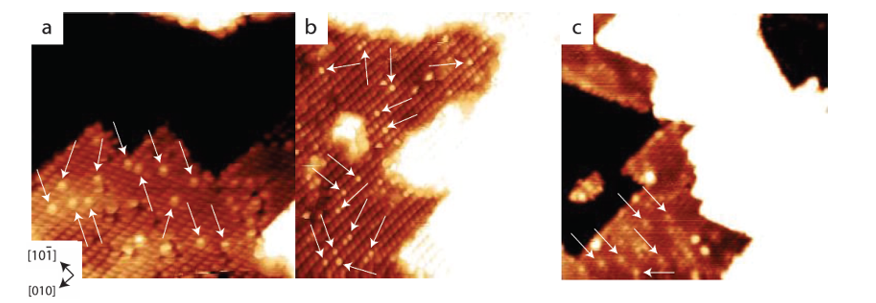
2.Oxygen vacancy (VO) mediated hydroxyl generation
Surface oxygen vacancies provide electrons, making adsorbed water molecules more prone to dissociation. After water molecules adsorb at the vacancy, two hydroxyl groups are generated, one of which fills the vacancy and the other hangs on the adjacent metal. This mechanism explains the phenomenon of significant increase in hydroxyl density under reducing or high-temperature conditions, and is closely related to changes in the coordination number of metal ions.
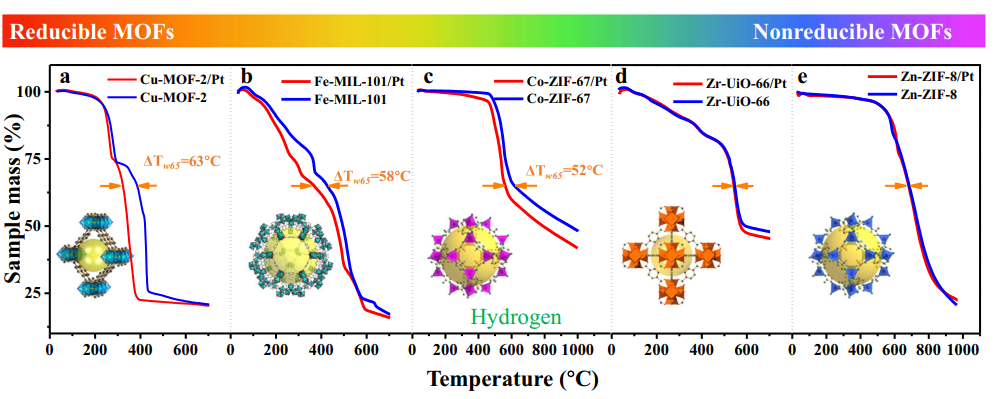
3.Hydrogen or hydrogen atom overflow
At the metal/oxide interface, H2 dissociates on the metal to form H ⁺/H ⁻, which then migrates to the surface of the metal oxide through hydrogen overflow and forms hydroxyl groups with surface oxygen. This process was directly observed in catalytic systems such as low-temperature CO oxidation, and hydrogen overflow significantly increased the rate of surface hydroxyl group generation.
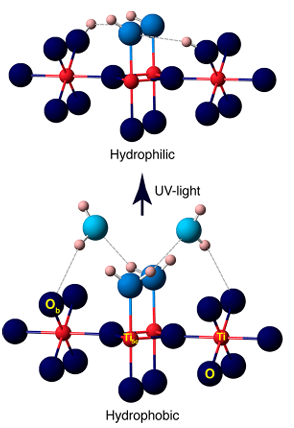
4.Photocatalytic/UV induced hydroxyl formation
UV light excites semiconductors such as TiO2 to generate electron hole pairs, which capture surface oxygen atoms to form O ⁻, and then react with adsorbed water molecules or hydroxyl groups to generate surface OH ⁻, accompanied by the production of hydroxyl radicals (· OH). Experiments have shown that UV irradiation generates additional oxygen vacancies on the surface of TiO2, which further react with water to generate more hydroxyl groups, leading to photo induced superhydrophilicity.
5.Formation of hydroxyl groups on the surface of aluminum oxide
A small amount of hydroxyl groups naturally exist on the surface of aluminum oxide, and water molecules dissociate and adsorb on these hydroxyl groups, producing new Al-OH. During atomic layer deposition (ALD), TMA (trimethylaluminum) undergoes coordination exchange with surface hydroxyl groups to form Al-O-Al bonds and release methane; Subsequently, the water pulse reacts again with Al-O bonds to regenerate surface hydroxyl groups, achieving cyclic regeneration of hydroxyl groups.
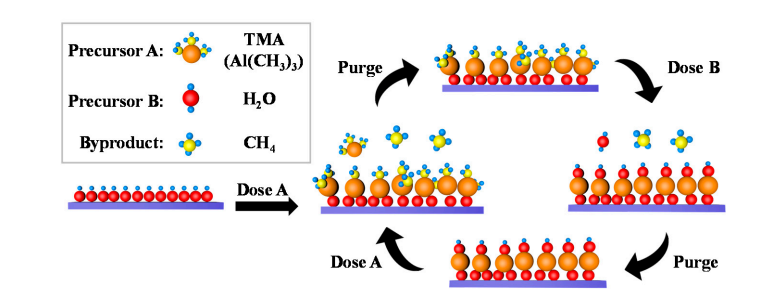
6.Surface reconstruction - Metal migration leads to hydroxyl aggregation
On the crystal surface of alumina or titanium oxide, local metal ions (such as Al3+) migrate to surface vacancies under high temperature or high hydrochemical potential, forming hydroxyl clusters of Al (OH) 3 or Ti (OH) 3 type. This reconstruction is accompanied by lattice distortion, which makes the adsorption of hydroxyl groups on adjacent water molecules more favorable, forming a high-density hydroxyl base layer.
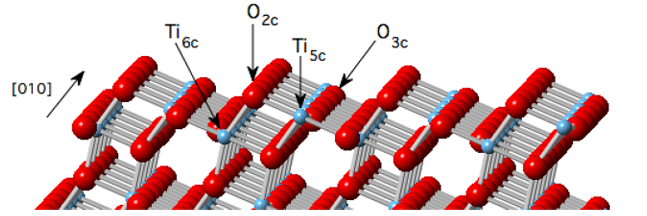
7. The hydrolysis mechanism of hydroxyl groups on silicon surface
At the Si-O-Si bridge bond, water molecules combine with the silicon oxygen bond through synergistic proton electron transfer, forming Si-OH groups. This process is particularly important in stress corrosion at the crack tip and surface hydrolysis of glass, and the enhancement of Si-OH tensile vibration was directly observed by experimental infrared spectroscopy.
SAT NANO is a best supplier of nano powder and micro particle in China, we can offer carbon nanotube powder, such as
MWCNT-COOH,
MWCNT-OH, if you have any enquiry, please feel free to contact us at sales03@satnano.com































