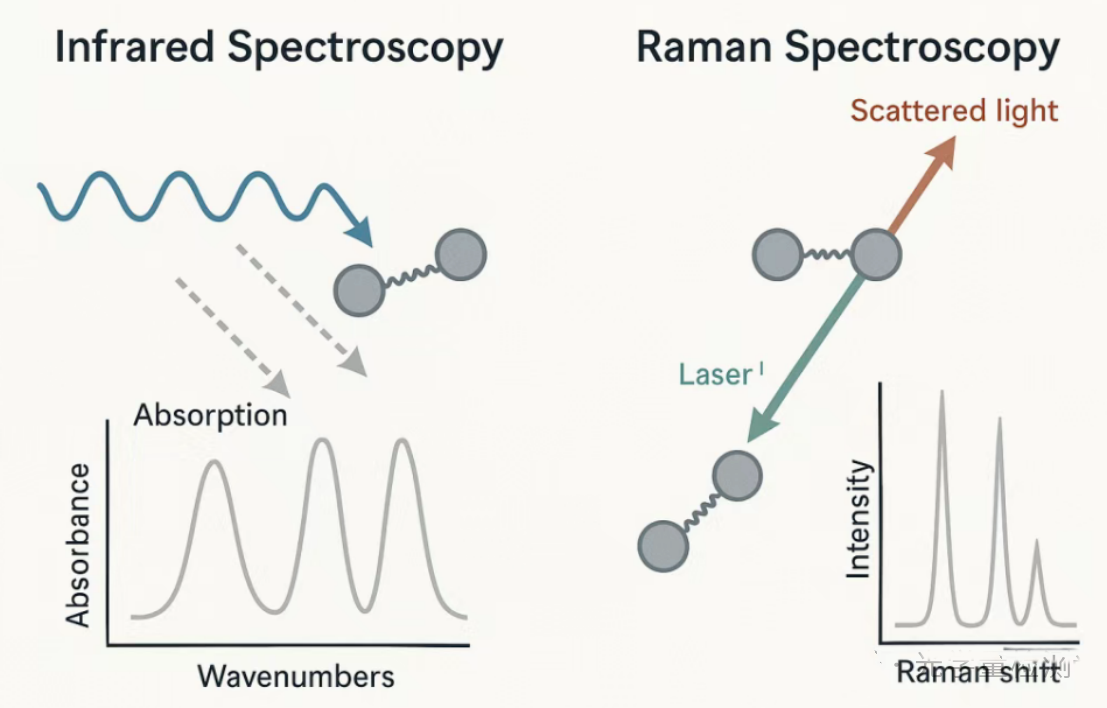
Infrared spectroscopy: It looks at how much light has been consumed. When a molecule absorbs light of a specific wavelength, we know what functional groups are inside it. Raman spectroscopy: It looks at how much light has been deflected. A laser beam is applied to analyze how much the light bounced back has changed, in order to determine the molecular structure.
What are the differences in principles?
This is the key to understanding the difference between the two. Let's use a metaphor to illustrate: imagine a beam of light as a group of people (photons) running towards a molecule.
Infrared spectroscopy (IR) - Among the group of people with "energy absorption", only those with a specific "weight" (energy) will be "eaten" (absorbed) by molecules. The instrument is watching from the side and says, "Oh, so many people of this weight are missing? It means there are bonds in the molecule that need this energy to vibrate." Key: The molecule must be able to "eat" this light, usually requiring an imbalance of positive and negative charges (dipole moment).
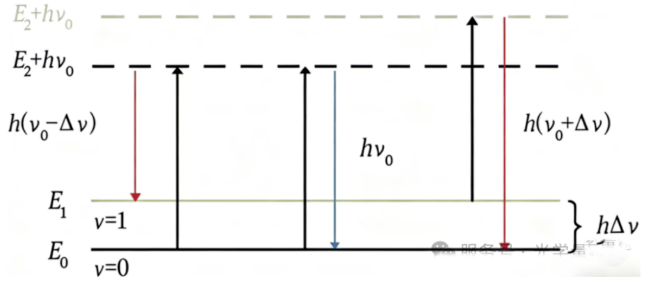
Raman spectroscopy - "Elastic collision" - We use a group of people (laser) with exactly the same weight to collide molecules. Most people are bounced back with their weight unchanged (Rayleigh scattering, useless signal). After a very small number of people collided, their weight changed slightly (Raman scattering), either becoming lighter (Stokes line) or heavier (anti Stokes line). The change in weight is the vibrational frequency of the molecule. The instrument is watching from the side and says, "Oh, has someone's weight changed so much? It means there is vibration at this frequency in the molecule." Key: When a molecule is hit, its electron cloud is easily distorted (polarization rate changes).
They can test which substance is best?
If you want to test the structural symmetry of non-polar bonds in carbon materials (such as graphene and carbon nanotubes) in aqueous solutions (for example, if you want to know if there are C=C double bonds in a bottle of oil, Raman spectroscopy is preferred)
If you want to test functional groups (such as - OH, - COOH) in proteins, plastics, and organic compounds, solid, liquid, and gas samples without water should be preferred for infrared spectroscopy
SAT NANO is bes supplier of nanopowder and micro particle in China, we can offer metal powder, alloy powder ,carbide powder and oxide powder, if you have any enquiry, please feel free to contact us at sales03@satnano.com



























