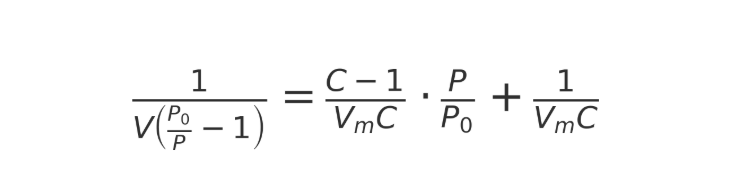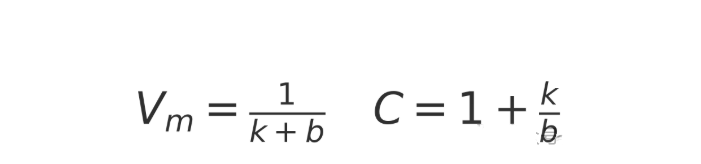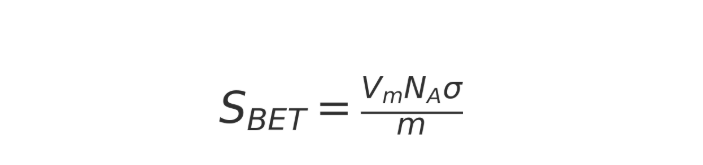In the fields of materials science, catalysis, energy and environment, specific surface area is one of the important parameters for measuring material performance. The adsorption efficiency of activated carbon, the activity of catalysts, and the energy storage performance of electrode materials are often closely related to their surface area. The most widely used surface area measurement method currently is BET specific surface area testing. This article will provide a detailed analysis of BET testing from several aspects, including principles, sample preparation, data processing, and precautions.
1、 The principle of BET testing
1.1 Adsorption phenomenon and specific surface area
On the surface of solid materials, gas molecules will adhere to the material surface in the form of physical adsorption, forming single or multiple molecular layers. When gas molecules reach equilibrium adsorption on the material surface, there is a certain relationship between the adsorption amount and the relative pressure of the gas. The BET theory was proposed based on this phenomenon.
1.2 BET equation
The BET (Brunauer Emmett Teller) theory was proposed in 1938, and its core is to derive a calculation method for specific surface area through the multi molecular layer adsorption behavior of gases on solid surfaces.
The BET equation is in the form of:
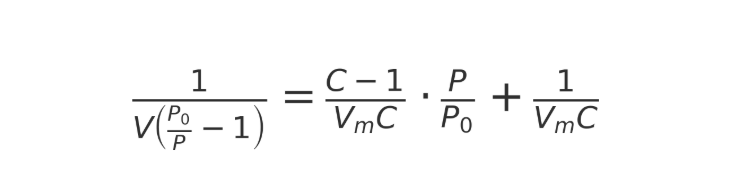
which:
(V) Adsorption capacity under relative pressure (P/Po)
(Vm): Single molecular layer adsorption capacity
(P) Adsorption pressure
(Po): Saturated vapor pressure
(C) Constant, reflecting the difference between adsorption heat and vaporization heat
After obtaining a series of adsorption data through experiments, a BET linear graph can be plotted (usually selecting (P/Po) in the range of 0.05-0.35), and Vm and C can be calculated from the slope and intercept, ultimately obtaining the specific surface area.
1.3 Gas selection
The commonly used adsorption media are:
Nitrogen (77 K) → Most common choice
Argon gas (87 K) → suitable for microporous materials
Carbon dioxide (273 K) → more suitable for ultramicropore measurement
2、 Sample preparation
BET testing requires extremely high pre-treatment of samples, and improper preparation can directly lead to result deviation.
2.1 Degassing treatment
Purpose: To remove moisture and impurity gases from the surface of the sample to avoid affecting the adsorption data.
Method: Vacuum or high-purity inert gases (such as helium and nitrogen) are commonly used for degassing.
Temperature selection: Set according to the material properties, generally within the range of 80 ℃ -350 ℃.
Polymer or organic skeleton materials: Low temperature (80-120 ℃) to avoid structural damage
Inorganic oxides and carbon materials: can be used at higher temperatures (200-350 ℃)
2.2 Sample size
Usually 50-300 mg of sample is required, depending on the instrument and material type. Powder materials should be evenly dispersed to avoid poor heat transfer caused by accumulation.
2.3 Precautions
Avoid air pollution: After degassing is completed, it should be transferred to the analysis end as soon as possible to reduce moisture absorption.
Maintain structural stability: For porous MOFs and other materials, temperature should be carefully controlled to avoid crystal collapse.
Repeatability: Try to test the same batch of samples under the same conditions as much as possible to improve data comparability.
3、 BET testing experimental steps
3.1 Obtaining adsorption desorption isotherms
Sample tube loading → fixed in the sample pool
Degassing treatment → Ensure surface cleanliness
Cold trap cooling → liquid nitrogen (77 K) or other cooling methods
Gradually increase the pressure → record the gas adsorption amount under different relative pressures
Complete cycle → Obtain complete adsorption desorption isotherm
3.2 BET interval selection
Usually fitted in the range of 0.05-0.35 P/P0
Must meet the BET consistency criterion
4、 Data Processing and Computation
4.1 Calculation of Single Molecular Layer Adsorption Capacity
By linearly fitting the BET equation, the slope (k) and intercept (b) can be obtained, and the following can be calculated:
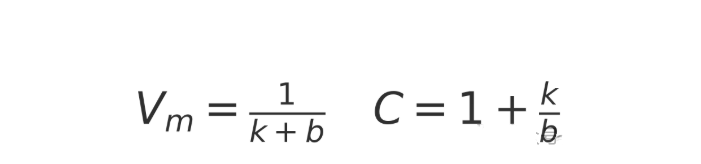
4.2 Calculation of specific surface area
Given the molecular cross-sectional area of a gas (nitrogen molecules are approximately 0.162 nm ²), then:
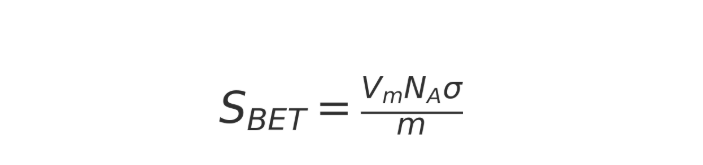
which:
(NA): Avogadro's constant
(σ) Gas molecule cross-sectional area
(m) : Sample quality
4.3 Analysis of adsorption desorption isotherms
In addition to BET specific surface area, information can also be obtained from isotherms and hysteresis loops:
Aperture distribution: calculated using BJH or DFT methods
Pore volume: estimated from the adsorption capacity under high relative pressure
Type of pore structure: I-VI isotherms and hysteresis curves correspond to different pore structures
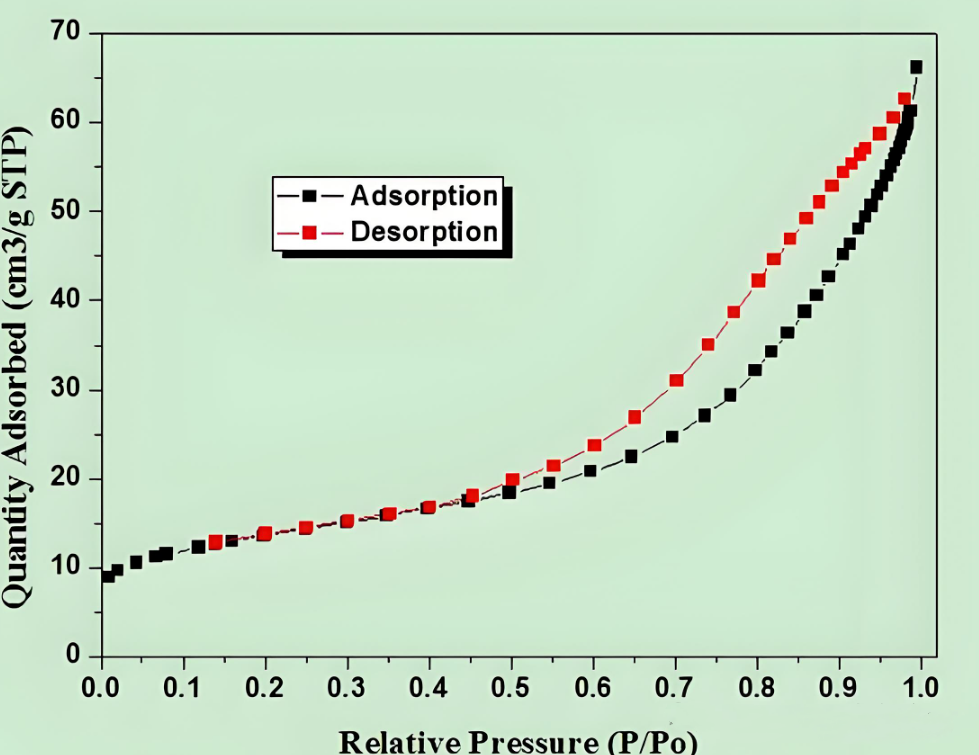
5、 Types and analysis of pore structures
In addition to specific surface area, BET testing combined with BJH, DFT and other methods can also provide pore size distribution information.
Micropores (<2 nm)
Mesopores (2-50 nm)
Macropores (>50 nm)
The aperture is greater than 50 nm.
In nitrogen adsorption, it usually exhibits a type II isotherm, and the adsorption capacity continues to increase with increasing pressure.
Macropores themselves do not contribute much to the specific surface area, but they play a role as "transmission channels" in composite porous structural materials, which can improve diffusion performance.
The aperture is between 2-50 nm.
It exhibits an IV type curve in the adsorption desorption isotherm, with a clear hysteresis loop.
Widely present in materials such as silica, alumina, mesoporous carbon, etc.
Advantages: Beneficial for molecular mass transfer, commonly used as a catalyst carrier.
Pore size less than 2 nm, providing ultra-high specific surface area.
Commonly found in activated carbon, zeolite, MOFs, etc.
The adsorption of nitrogen at 77 K may be limited by diffusion, and CO ₂ adsorption is needed to supplement the measurement.
6、 Common problems and precautions
Improper selection of BET interval: Too low or too high relative pressure can lead to fitting deviation.
Excessive or insufficient degassing:
Excessive → collapse of material structure
Insufficient → Surface residual impurities, adsorption capacity is falsely high
Excessive sample activity: Some catalysts may interact with nitrogen during the testing process, which requires special attention.
Difficulty in comparing results: Different laboratories may use different pre-treatment conditions, so when publishing data, the degassing temperature, time, and adsorbed gas type should be indicated.
7、 Application areas of BET testing
Catalyst development
The larger the specific surface area, the more active sites there are, and the catalytic activity is usually higher.
energy materials
The energy storage performance of electrode materials for lithium batteries and capacitors is closely related to their specific surface area and pore structure.
Adsorbents and separation materials
The adsorption performance of activated carbon, zeolite, MOFs, etc. directly depends on the specific surface area.
environmental governance
High specific surface area materials are required for the adsorption and removal of pollutants such as VOCs and heavy metal ions.
BET specific surface area testing, as a classic and practical characterization method, has been applied in the field of materials science for over 80 years. Through reasonable sample preparation, interval selection, and data processing, researchers can obtain accurate surface area and pore structure information, providing solid data support for material design and performance optimization.
SAT NANO is a best supplier of nano and micro material in China, we can supply metal powder, carbide powder, oxide powder and so on, we are not only supply product, but also supply different test service like SEM, BET test, if you have any enquiry, please feel free to contact us at sales03@satnano.com